A remarkably large and sometimes confusing set of laws, regulations, and internal policies apply to the research, technical services, and educational activities that our faculty and other JHU employees pursue. JHURA and other JHU offices endeavor to identify instances in which those laws, regulations, and policies require some kind of action on our part to insure compliance. Below, you will find links to resources that will enable you to partner with JHURA to anticipate and address compliance issues connect with your research or other work at JHU.

If you are hoping to pursue research that will involve animals, you should know that there are all manner of restrictions upon how they may be lawfully transported, handled, housed, and otherwise used for scientific and educational applications. There are also external, non-governmental organizations that can be highly critical of how animals are used at a research university, even if those uses are authorized by law. Our Animal Care and Use page provided below leads to resources that can help you to determine if you are prepared to lawfully use animals in your work.

If you are a JHU employee who has significant, personal investments (financial or otherwise) in organizations that are doing business with JHU, you may be subject to laws and regulations that restrict the type of involvement that you may have with those outside organizations as a JHU employee. Our Conflict of Interest page provides resources that can help you determine whether work that you would like to do as a JHU would be regarded as a conflict of interest/commitment.

Multiple laws and regulations require that we collect, protect, retain, and share data associated with our research according to certain standards. For example, personal health information that is collected must be protected from unauthorized access by certain third parties, and our researchers often receive research funding upon the condition that their results are made public within a certain period of time after the conclusion of the research. Our Data Stewardship page provides that describe the multiple ways in which the U.S. Government requires that we be responsible stewards of the information that we collect and use in the course of our research.

If you are performing work that involves foreign countries (e.g., visiting them or sending items, information or funds to them, physically or electronically, directly or indirectly) or their citizens (even when they are working at JHU), your project may be subject to one or more bodies of regulation that restrict the exchanges and other transactions that we can enter into with certain countries and their citizens. Our Export Control-Facility Security page provides resources that can help you determine whether some aspect of your work will require a license before you enter into certain transactions with foreign countries and citizens. Please also follow the link below if you are contemplating work that is expected to involve sensitive information designated as “classified” by the U.S. Government.

The General Data Protection Regulation (GDPR) standardizes data protection law across all 28 European Union (EU) countries and imposes strict new rules on controlling and processing of personal information. It went into effect as of May 25, 2018. Our GDPR page provides guidance and resources to help you determine if GDPR applies to your research.

Sometimes our researchers need to ask ordinary people if they would be willing to participate in studies that can help us determine whether certain drugs or other treatments in development are effective. The steps that we must take to involve human subjects in our research are highly regulated, and for good reason. However, one does not have wait very long to see in the news a story about an institute or hospital somewhere in the world that failed to create or adhere to practices designed to ensure compliance with such regulations and the safety of human subjects. Our Human Subjects page provides resources that can help you to understand the laws and other standards that apply to the involvement of human subjects at JHU and the practices that we have decided to use to ensure our compliance with them.

The Johns Hopkins Open Access Policy requires all full-time faculty to make a version of their peer-reviewed journal articles openly available. This policy aligns with Hopkins’ mission of Knowledge for the World. The policy requires that articles accepted for publication on or after July 1, 2018, where the sole or corresponding author is a JHU full-time faculty, be made openly available.

The U.S. Government, private organizations, and the general public expect our research to show the highest levels of integrity from the time we submit proposals and until we report their results. Our Research Integrity page provides resources that identify the regulations, guidelines and professional norms that one should follow in order to earn the confidence of our sponsors and the general public. You can also find resources on available training in the responsible conduct of research, and contact information for where to report suspected research integrity concerns.

Some kinds of research are controversial, such as that which is performed through the use of stem cells. The regulatory environment associated with controversial research often swings with changes in the U.S. political environment, which means that regulations and standards can change from administration to administration. If you are contemplating research with stem cells, then please visit our Stem Cell Research page to understand how your work might be impacted by the current regulatory environment and JHU’s response to it.
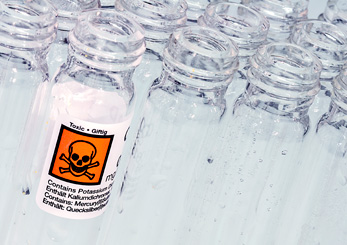
Some kinds of research require the handling of materials that are known to cause disease or other harm to humans and other animals. For example, a lab may be working with harmful bacteria, powerful chemicals or radioactive material. Our Workplace and Lab Safety page provides resources that can help you to determine whether any materials associated with your research may be subject to restrictions on their use, including training that may be required before you receive them, how you must handle them when they are on JHU property and how they must be disposed of.