Introduction
In February 2022, we announced the release of the new NIH Data Management and Sharing Policy, (DMSP) which was effective on January 25, 2023. This new policy requires submission of a Data Management and Sharing Plan as part of all proposals for research that will generate scientific data.
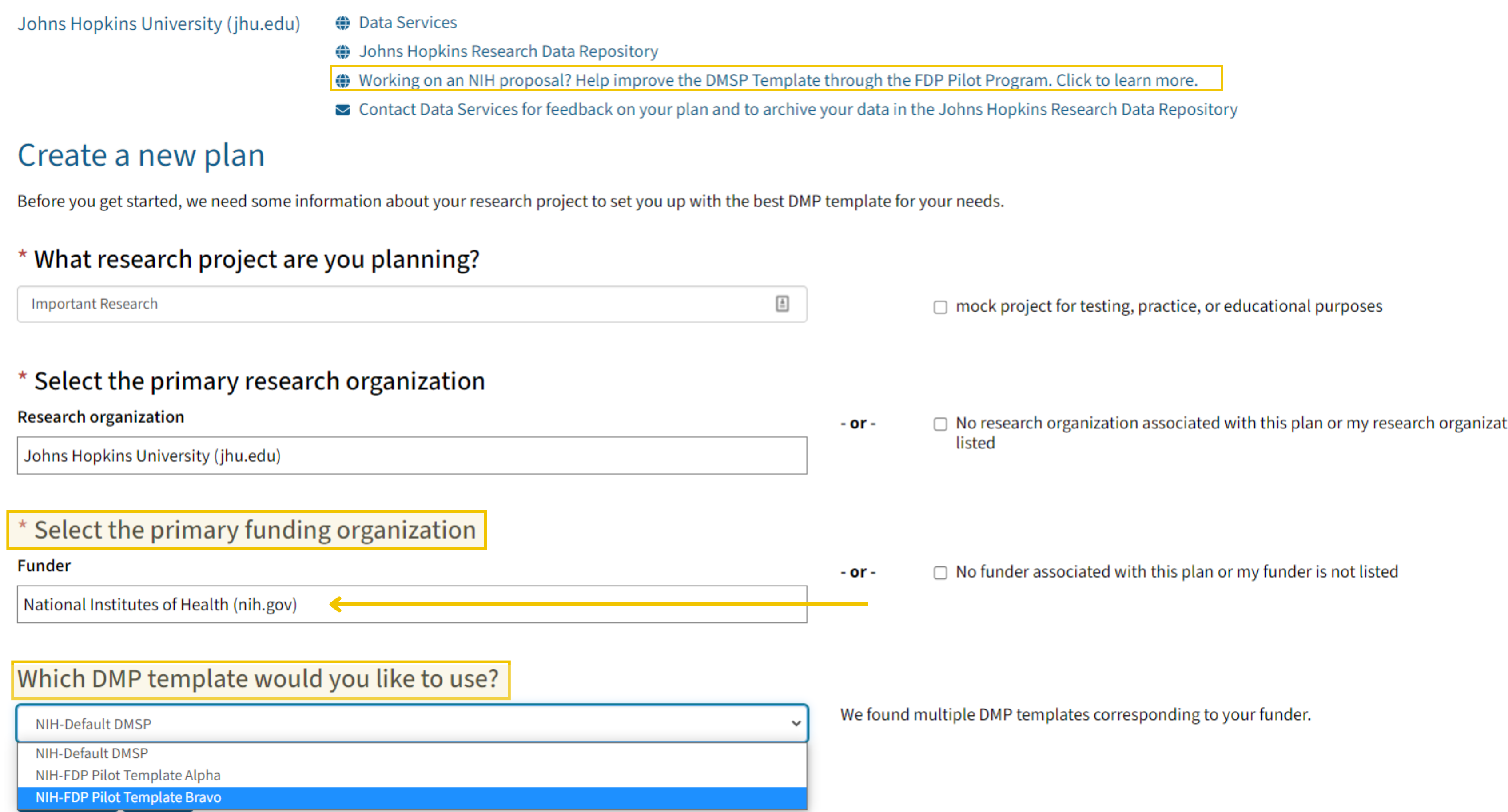
Johns Hopkins University is participating in the Federal Demonstration Partnership (FDP) NIH DMSP Template Pilot, which is testing the effectiveness and usability of two DMSP templates developed in collaboration with representatives from FDP, including participating NIH Institutes and Centers (ICs). The ultimate goals of the pilot are to harmonize DMSP requirements across NIH ICs and programs and to mitigate the administrative burden for researchers associated with DMSP development and implementation. Further information about the FDP DMSP Template pilot is available here FDP website.
As a reminder, JHURA/ORA ,Data Services, and Welch Medical Library each have online resources to help you navigate the requirements of the new NIH Policy
Please let us know if you have any questions or concerns. We hope you will add your voice to this important opportunity to shape the development of future NIH required formats!